
Related Attributes
Product details
iodine powder Usage and Synthesis.
Iodine deficiency can lead to the formation of goiter, in which the gland surrounding the trachea in the neck becomes enlarged. There are other causes of goiter, including thyroid cancer. Iodine deficiency can also cause neonatal cretinism (hypothyroidism in infants), which may lead to mental retardation unless the subject takes thyroid hormone throughout his life.
Uses of iodine powder.
It is mainly used to make iodide, pesticides, feed additives, dyes, iodine, test paper, drugs, etc. It is used to prepare equivalent solvents, determine iodine value, and calibrate the concentration of sodium thiosulfate solution. The solution can be used as a disinfectant. It is used to prepare iodine and thinning solution in phototypesetting.
One of the most important uses of iodine is to treat hypothyroidism, a disease caused by a lack of iodine in the thyroid gland.

Physical properties of iodine.
Iodine in its pure state is a black solid that sublimes (changes from a solid state to a gas without passing through a liquid state) at room temperature. Iodine readily forms a non-metallic diatomic molecule (I2) and is the heaviest of the naturally occurring halogens. Iodine is the least reactive of the five halogens. Iodine compounds are less reactive than the other halogens, which readily displace it from its iodide. Iodine has some metal-like properties. It dissolves readily in chloroform, carbon tetrachloride, or carbon disulfide, forming a beautiful purple solution. It is only slightly soluble in water. Iodine compounds are important in organic chemistry and are very useful in medicine. It has 42 isotopes and isomers. Only one stable isotope, iodine-127, is found in nature. The artificial radioactive isotope iodine-131 has a half-life of 8 days.
Why choose us?

HRK Factory

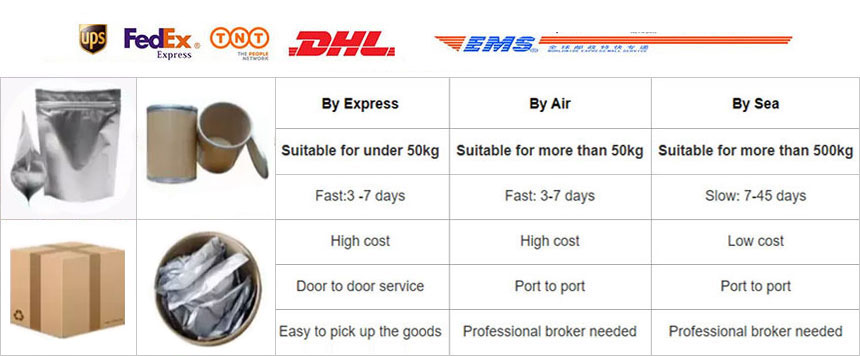
About Shipping
Pharmaceutical Intermediate manufacturers
©2023 Xi'an Henrikang Biotech Co., Ltd.,