





Related Attributes
Product details
Sodium fluoride powder Colorless bright crystals or white powder, specific gravity 2.25, melting point 993°C boiling point 1695°C. Soluble in water (solubility 10°C 3.66, 20°C 4.06, 30°C 4.22, 40°C 4.4, 60°C 4.68, 80°C 4.89, 100°C 5.08), hydrofluoric acid, slightly soluble in alcohol.Sodium fluoride CAS 7681-49-4 The aqueous solution is weakly alkaline, dissolved in hydrofluoric acid to form sodium hydrofluoric acid, and can corrode glass. It can corrode glass. Toxic!
Colorless cubic or tetragonal crystal. Sensitive to moisture. Solubility in water (g/100ml): 4 at 15℃, 4.3 at 25℃, 5 at 100℃, Sodium fluoride Raw Materials Insoluble in ethanol. Aqueous solution partially hydrolyzed to alkaline reaction. The pH of newly prepared saturated solution is 7.4.

Its aqueous solution can make glass hairy, but Sodium fluoride Raw Materials dry crystals or powder can be stored in glass bottles. Relative density 2.78. melting point 993℃. Boiling point 1695℃. Moderately toxic, LD50 (rat, oral) 0.18g/kg. Sodium fluoride Raw Materials has strong irritation.
Applications / Functions of Sodium fluoride powder
Physicochemical Property of Sodium fluoride powder
Relative density 2.558(41/4℃), melting point 993℃, boiling point 1695℃. (Relative density 2.79, melting point 992 ℃, boiling point 1704 ℃ [3]) can be soluble in water (15 ℃, 4.0g/100g; 25 ℃, 4.3g/100g), Sodium fluoride CAS 7681-49-4 can be soluble in hydrofluoric acid, insoluble in ethanol. Aqueous solution is alkaline (pH=7.4). Toxic (injury to nervous system), LD50180mg/kg (rat, oral), 5~10g lethal. Properties: colorless or even white crystalline powder, or cubic crystal system microfine crystals, odorless.

Colorless shiny crystals or white powder, belonging to the tetragonal crystal system, with positive hexahedral or octahedral crystals. Sodium fluoride powder is slightly soluble in alcohol; soluble in water, aqueous solution is acidic, dissolved in hydrofluoric acid to become sodium hydrogen fluoride.
production methodprocess of Sodium fluoride powder
Fluorite, soda ash and quartz sand are calcined at high temperature (800-900℃), then leached with water, then evaporated, crystallized and dried to get the finished product.CaF2+Na2CO3+SiO2→2NaF+CaSi03+C02↑.
Sodium fluoride powder is obtained by neutralizing hydrofluoric acid with soda ash or caustic soda, 2HF+Na2CO3→2NaF+H2O+CO2↑Dissolve soda ash with mother liquor in the neutralization pot, and then add 30% of hydrofluoric acid and neutralize it until the pH value is 8~9, and there is CO2 gas escaping, Hydrofluoric acid often contains fluosilicic acid impurity, and after neutralization, it generates sodium fluosilicate. 90 ~ 95 ℃: heating 1h, sodium fluorosilicate is decomposed: Na2SiF6 + 2Na2CO3 → 6NaF + SiO2 + 2C02 ↑ and the process of neutralization pH value shall not be lower than 8, otherwise sodium fluorosilicate is difficult to be decomposed by the alkali, and the neutralization solution is left to stand for 1h, and the clear liquid is concentrated and then cooled to precipitate sodium fluoride crystals, and then centrifugal separation, drying, crushing to get Sodium fluoride. CAS 7681-49-4
Sodium fluorosilicate is 99.87% of sodium fluorosilicate produced by wet process phosphoric acid and phosphorus fertilizer by-products according to conventional methods. In 84 ~ 95 ℃ and 0.15MPa under the conditions of reaction 160 ~ 180min, excess 5% ~ 8% of alkaline solution (pH value of 8 ~ 9), the generation of sodium fluoride. Na2SiF6 + 2Na2Co3 → 6NaF + SiO2 + 2C02 ↑ neutralized liquid static clarification, the clear liquid by evaporation and concentration, cooling and crystallization, separation and washing with hot water at 50 ~ 60 ℃. Sodium fluoride powder was obtained by indirect drying.

Neutralization method in the reactor to add soda ash (dissolved with mother liquor), and then will be formulated 30% hydrofluoric acid solution pumped into the reactor, to Ph value of 8 ~ 9, that is, stop adding acid, stirring to make the reaction to Ph> 8 ~ 9, and then add acid to Ph8 ~ 9, such as no carbon dioxide bubbles escaping that is the end point. The reaction liquid is left to stand for 1h, after centrifugal separation, drying, crushing, to produce sodium fluoride finished products. Its 2HF + Na2CO3 → 2NaF + H2 + CO2 ↑ melting leaching method will be fluorite, soda ash and quartz sand calcined at 800 ~ 900 ° C, and then water leaching, filtration, the filtrate and then by evaporation, crystallization, drying, the production of Sodium fluoride powder products. Its CaF2+Na2CO3+SiO2→2NaF+CaSiO3+CO2↑Sodium fluorosilicate method will be phosphate fertilizer factory exhaust gas produced by sodium fluorosilicate and sodium carbonate according to the 1:2 molar ratio of mixing, the reaction at about 80 ℃, the precipitation of sodium fluoride crystals and silica gel, and then added to the sodium hydroxide, the silica gel into a soluble silicate (Na2O-2SiO2~Na2O-3SiO2) Dissolved. Then filtered and dried to produce Sodium fluoride Raw Materials . Its Na2SiF6+2Na2CO3+H2O→6NaF+SiO2-H2O+2CO2
Why choose us?

HRK Factory

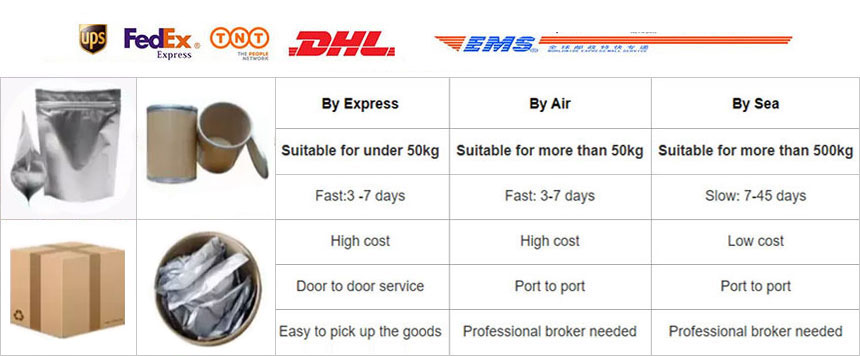
About Shipping
Pharmaceutical Intermediate manufacturers
©2023 Xi'an Henrikang Biotech Co., Ltd.,