





Related Attributes
Product details
Ethyl acetate Liquid, also known as ethyl acetate, is the hydroxyl group in acetic acid replaced by ethoxy group to produce compounds, the structure of the simple formula for CH3COOCH2CH3. pure Ethyl acetate CAS 141-78-6 is colorless transparent liquid with aromatic odor, melting point: -83.6 ℃, boiling point: 77.06 ℃, relative density (water = 1): 0.894-0.898, relative vapor density (air = 1): 3.04, has a strong odor. Relative density (water=1): 0.894-0.898, relative vapor density (air=1): 3.04, has a strong ether-like odor, clear, slightly fruity wine aroma, easy to diffuse, not persistent.

Ethyl acetate Raw Materials slightly soluble in water, soluble in alcohol, ketone, ether, chloroform and most other organic solvents.Ethyl acetate CAS 141-78-6 is a kind of fine chemical products with wide range of uses, with excellent solubility, fast drying, wide range of uses, Ethyl acetate Liquid is a very important Ethyl acetate liquid is a very important organic chemical material and excellent industrial solvent
Applications / Functions of Ethyl acetate Liquid
Ethyl acetate Liquid is one of the most widely used fatty acid esters, it is a fast-drying solvent with excellent solvency and is an excellent industrial solvent. It can be used in nitrate fiber, ethyl fiber, chlorinated rubber and vinyl resins, cellulose acetate esters, cellulose butyl acetate and synthetic rubber, and can also be used in liquid nitrocellulose inks for photocopiers. Can be used as a solvent for adhesives, spray paint thinner;
Ethyl acetate CAS 141-78-6 can be used as a cleaning agent in the textile industry, in the food industry as a special modified alcohol flavor extractant, also used as a pharmaceutical process and organic acid extractant. In the fragrance industry is an important fragrance additive, can be used as a component of the flavoring agent.Ethyl acetate Raw Materials is also the raw material for the manufacture of dyes, drugs and spices. Ethyl acetate is an efficient solvent for many types of resins and is widely used in the production of inks and artificial leather.

Extractant, extract many compounds (phosphorus, tungsten, arsenic, cobalt) from aqueous solutions, used as a solvent for crystallization in organic analysis, used as a standard for calibrating thermometers when separating sugars, checking bismuth, gold, iron, mercury, oxidizers, and platinum, determining bismuth, boron, gold, iron, molybdenum, platinum, potassium, and thallium. Biochemical research, protein sequence analysis. Environmental protection, pesticide residue analysis. Organic synthesis. Flavor manufacturing.
Physicochemical Property of Ethyl acetate Liquid
Colorless, flammable liquid with fruity smell, Ethyl acetate Raw Materials miscible with ether, alcohol, halogenated hydrocarbon, aromatic hydrocarbon and other organic solvents, slightly soluble in water.
Production methodprocess of Ethyl acetate Liquid
1, esterification method by acetic acid and ethanol in the presence of sulfuric acid directly esterified, the production process has continuous and intermittent points.
(1) Intermittent process. Add acetic acid, ethanol and a small amount of sulfuric acid into the reactor, heating reflux 5-6h. Then evaporate the ethyl acetate, and wash with 5% brine, sodium hydroxide and sodium chloride mixed solution and neutralize to PH=8. Then wash with calcium oxide solution, add anhydrous potassium carbonate dry. Finally distill, collect the 76-77 ℃ fraction, that is, Ethyl acetate Raw Materials .
(2) Continuous process: 1:1.15 (mass ratio) of ethanol and acetic acid are continuously fed into the kettle of esterification tower, and the esterification reaction is carried out under the catalytic effect of sulfuric acid at 105-110°C. The resulting ethyl acetate and water are then washed with calcium oxide solution and dried with aqueous potassium carbonate. The generated ethyl acetate and water are distilled from the top of the tower in the form of azeotrope, and after condensation and stratification, the upper ester is partly refluxed and the rest goes into the crude product tank, and the lower water is given up after recovering the ethyl acetate. The crude ester is stripped of a small amount of water by the de-boiling tower before entering the refining tower, and Ethyl acetate Raw Materials can be obtained at the top of the tower . This process is better than the gap method.

2, acetaldehyde method acetaldehyde in ethanol aluminum catalyzed to generate ethyl acetate, acetaldehyde, ethanol aluminum and so on continuously added to the two tandem reactor, at 0-20 ℃ for the reaction, the second reactor outlet conversion rate of up to 99.5% or more, and then distillation of Ethyl acetate Liquid. yield of 95% -96%, this process is more economical.
Why choose us?

HRK Factory

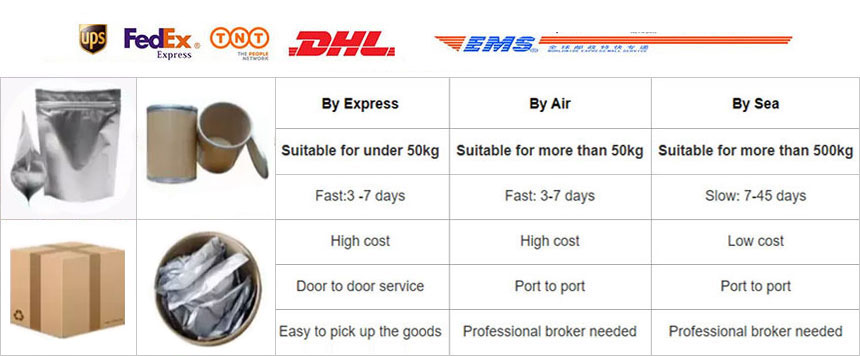
About Shipping
Pharmaceutical Intermediate manufacturers
©2023 Xi'an Henrikang Biotech Co., Ltd.,