





Related Attributes
Product details
Tetrachloroethylene, an organic chemical, is widely used in dry cleaning and metal degreasing, as well as in the manufacture of other chemicals and consumer products. It is a non-flammable liquid at room temperature. It evaporates readily and has a pungent sweet odor. Many people can smell tetrachloroethylene when the air contains one part per million. The first time Michael Faraday heated hexachloroethane in 1821, it decomposed into tetrachloroethylene and chlorine gas. Tetrachloroethylene is toxic and is a central nervous system depressant, causing headaches, nausea, vomiting, and even coma. The danger of tetrachloroethylene to It can be poisoned by absorption through the respiratory tract, digestive tract and skin. Tetrachloroethylene is less toxic than trichloroethylene, and its anesthetic effect is weaker. Tetrachloroethylene is less toxic than trichloroethylene.

Pharmacokinetics of Tetrachloroethylene:
Tetrachloroethylene is insoluble in sugar, glycerol and protein, slightly soluble in water (0.015 at 25°C), and miscible with ethanol, ether, chloroform, benzene and chlorinated organic solvents. Not hydrolyzed. In In the absence of air, moisture and catalyst, it is still stable even when heated to 500℃. When hydrogenated, it can produce tetrachloroethane. When chlorinated, hexachloroethane is produced. Tetrachloroethylene can also It can react with bromine to produce monobromotrichloride or dibromodichlorine compounds. It can also react with hydrogen fluoride under the action of catalytic agents. In the presence of light, air and water for a long time In the presence of light, air and water, it slowly decomposes into trichloroacetaldehyde and phosgene, and corrodes iron, aluminum, zinc and other metals, which can be inhibited by adding stabilizers. If the presence of activated carbon, heated to 700 ℃ decomposition into Decompose into hexachlorobenzene and hexachloroethane. Tetrachloroethylene can be oxidized by strong oxidizing agents. Tetrachloroethylene can be violently reacted with barium powder, beryllium powder, lithium chips, dinitrogen tetroxide and sodium hydroxide. Chemical reaction.

Clinical Application of Tetrachloroethylene:
Tetrachloroethylene is widely used as an organic solvent, dry cleaning agent, metal degreasing solvent, and as an anthelmintic. Tetrachloroethylene is used as a fatty extractant, fire extinguishing agent It is also used in the synthesis of trichloroethylene and fluorinated organic compounds. Tetrachloroethylene is mainly used in industry as a solvent, organic synthesis, metal surface cleaner and dry cleaning agent, desulfurizer, and thermal compound. In industry, tetrachloroethylene is used as a solvent, organic synthesis, metal surface cleaner and dry cleaning agent, desulfurization agent, and heat transfer medium. It is used as a medical anthelmintic. It is also used as an intermediate in the production of trichloroethylene and fluorinated organic compounds. The general population is exposed to low concentrations of tetrachloroethylene through the atmosphere, food and drinking water. The general population may be exposed to low concentrations of tetrachloroethylene through the atmosphere, food and drinking water. Tetrachloroethylene is also an anthelmintic and is effective in treating hookworm infections and in killing hookworms of the duodenum and American hookworm. However, it can occur However, side effects such as dizziness, headache, nausea, sleepiness and Moet-like effects can occur.

Why choose us?

HRK Factory

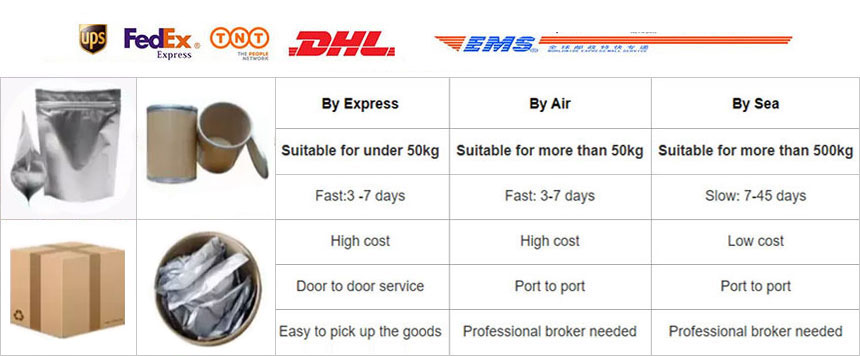
About Shipping
Pharmaceutical Intermediate manufacturers
©2023 Xi'an Henrikang Biotech Co., Ltd.,