





Related Attributes
Product details
Ferrous chloride Powder, chemical formula FeCl2, molecular weight 126.75, white rhombic crystals with CdCl2 structure. Melting point 672℃, Boiling point 1030℃, Relative density 3.16225.Ferrous chloride CAS 7758-94-3 White crystals obtained by sublimation in the hydrogen chloride gas stream at 700℃ are highly hygroscopic.
Ferrous chloride Raw Materials dihydrate is slightly light green colorless monoclinic crystal, relative density 2.358, easy to oxidize in the air, 150~160℃ decompose to produce a hydrate, 220℃ lose all the water of crystallization.

Ferrous chloride CAS 7758-94-3 tetrahydrate is blue-green monoclinic crystal, hygroscopic, relative density 1.93, very soluble in water and ethanol, soluble in acetone, slightly soluble in benzene, insoluble in ether. Very easy to oxidize in humid air.
Applications / Functions of Ferrous chloride Powder
Ferrous chloride CAS 7758-94-3 is used in metallurgy and medicine manufacturing. Used as analytical reagent, water treatment purifying agent, mordant, unleaded gasoline auxiliary and reducing agent.
Physicochemical Property of Ferrous chloride Powder
Ferrous chloride Powder Ferrous chloride is white or gray-green crystal at room temperature. It is easy to absorb moisture. Easily oxidized in the air and gradually become yellow. Relative density d4253.162. Melting point 677℃.

Boiling point 1023℃. Heat of melting 43.01kJ/mol, Ferrous chloride Raw Materials Sublimation at about 700℃ in hydrogen chloride gas stream. Soluble in water, methanol, ethanol, slightly soluble in acetone and benzene, insoluble in ether.
Production methodprocess of Ferrous chloride Powder
Ferrous chloride Raw Materials is obtained by heating iron in dry hydrogen chloride gas stream at a temperature controlled below 500℃, or reducing anhydrous ferric chloride with dry hydrogen at 300~350℃.
Take ferric chloride as raw material and heat it with chlorinated benzene at 125℃ for 30min, and absorb the generated hydrogen chloride with water. Continue heating at 130~139℃ for 2h, then the precipitate changes from black to brown. The viscosity decreases, under the condition of air isolation, filter the solid, wash with chloroform and benzene, can be produced Ferrous chloride Powder.

In a hard glass tube quickly put anhydrous ferric chloride, from one end of the tube through the dry hydrogen, fully replaced to remove the air inside the tube, the reaction tube is heated, the other end of the tube that is a large number of hydrogen chloride discharged, can be absorbed into dilute hydrochloric acid with water. When the rate of release of hydrogen chloride slows down, ferric chloride into white crystals, that the reaction is complete, stop heating, and in the weak hydrogen flow to bring the product in the tube to room temperature.
The reaction tube will be closed and stored, and then quickly loaded into the packaging container to produce Ferrous chloride CAS 7758-94-3, the reaction equation: 2FeCl3 + H2 → 2FeC12 + 2HCl
Why choose us?

HRK Factory

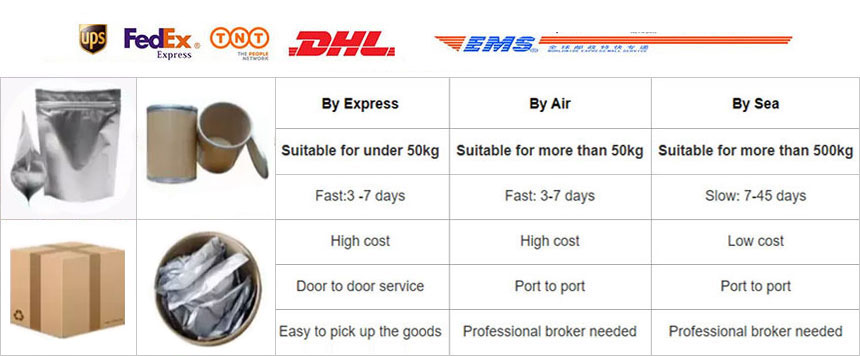
About Shipping
Pharmaceutical Intermediate manufacturers
©2023 Xi'an Henrikang Biotech Co., Ltd.,