

Related Attributes
Product details
description of Hexafluorosilicic acid.
Hexafluorosilicic acid is a chemical reagent used in experiments. It can be used for metal surface treatment, drinking water fluoridation, wood antiseptic treatment, glass production, ceramic materials, etc. The important derivatives of hexafluorosilicic acid include aluminum fluoride, cryolite, and sodium fluoride.

Hexafluorosilicic acid water fluoridation.
Fluoridation of water is carried out by using either hexafluorosilicic acid, which is a solution usually containing 20 % w w H 2 SiF 6 ( 15.8 % w w F ) , or disodium hexafluorosilicate, which is supplied as a powder usually containing at least 98 % NaSiF 6 ( 59.4 % w w F ) (Table 7.5).

Chemical properties of hexafluorosilicic acid.
Fluorosilicic acid is a coordination acid with strong acidity. The pH is 1.6 at a concentration of 0.03 mol/L (excluding decomposition, the acidity is similar to that of nitric acid). If stored in a plastic container, it is stable in a dilute solution at room temperature. The complex ion fluorosilicate (SiF62-) is very stable. The presence of six Fs and the very symmetrical structure allow the negative charge to be well dispersed, allowing fluosilicic acid to release hydrogen ions well. Its salts have special solubility. Potassium and barium salts are insoluble, rubidium salts are slightly soluble, sodium salts, lithium salts and ammonium salts are soluble, and cesium salts and ferrous salts are very soluble. It decomposes under heat to release toxic fluoride gas. It is highly corrosive.

Product Method of hexafluorosilicic acid.
Silica powder acid hydrolysis method: First add hydrofluoric acid to the lead lining acid hydrolysis equipment, and then gradually add silica powder to react. After the reaction is completed, add rice husk ash to neutralize the free hydrofluoric acid and play the role of bleaching solution. Filter the solution, add an appropriate amount of yellow lead powder (PbO) to the filtered clear liquid to remove the sulfate brought in by the hydrofluoric acid, and then filter it to obtain the finished fluosilicic acid. Its 6HF+SiO2→H2SiF6+2H2O
Why choose us?

HRK Factory

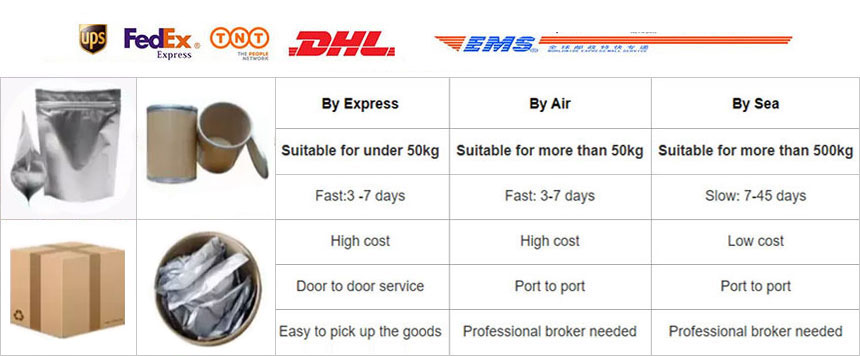
About Shipping
Pharmaceutical Intermediate manufacturers
©2023 Xi'an Henrikang Biotech Co., Ltd.,