





Related Attributes
Product details
Hydrofluoric acid is an aqueous solution of hydrogen fluoride gas, a clear, colorless, fuming corrosive liquid with a strong irritating odor. Melting point -83.3℃, boiling point 19.54, flash point 112.2℃, density 1.15g/cm³. Easily soluble in water, ethanol, slightly soluble in ether. Because of the relatively strong bonding ability between hydrogen and fluorine atoms, which makes hydrofluoric acid in water Theoretically, hydrofluoric acid in low concentration is a weak acid and belongs to inorganic acid because it cannot be completely ionized in water. It is extremely corrosive and can strongly corrode metals, glass and objects containing silicon. If inhaling the vapor or coming in contact with the skin can cause incurable burns. If the skin contact immediately with a large amount of water for a long time to thoroughly rinse, as soon as possible to dilute and wash away the hydrofluoric acid Dilute and rinse off the hydrofluoric acid as soon as possible.

Pharmacokinetics of Hydrofluoric Acid:
Hydrogen fluoride is irritating and corrosive to clothing, skin, eyes, respiratory tract, and digestive tract mucous membrane, and fluoride ions can combine with its calcium and magnesium ions when entering blood or tissues, making them Fluoride ions into the blood or tissue can be combined with calcium and magnesium ions, making them insoluble or slightly soluble calcium fluoride and magnesium fluoride, if the amount is large, directly block the blood vessels, directly or indirectly affect the function of the central nervous system and cardiovascular system, leading to low blood Fluoride ion can also combine with hemoglobin to form fluorohemoglobin, which can inhibit succinate dehydrogenase and reduce oxygenation and affect cellular respiration. The fluoride ion can also combine with hemoglobin to form fluorohemoglobin, inhibit succinate dehydrogenase, reduce oxygenation and affect cellular respiration. Commercially available usual concentration: 40% mass fraction of solute, industrial grade; 40% mass fraction, electronic grade. Highly hazardous poison. The density at its most concentrated is 1.18g/cm3. As the mass fraction of HF solution increases, the corrosion rate of HF on carbon steel increases and then decreases.

Clinical Application of Hydrofluoric Acid:
Hydrofluoric acid plays an important role in the purification of aluminum and uranium because of its ability to dissolve oxides. Hydrofluoric acid is also used to etch glass for engraving patterns, marking scales and text. It is used in the semiconductor industry to remove oxides from silicon surfaces, in oil refineries as a catalyst for the alkylation of isobutane and n-butene, and in stainless steel to remove oxygen-containing impurities from surfaces. It is also used in the semiconductor industry to remove oxides from silicon surfaces, in oil refineries as a catalyst for the alkylation of isobutane and n-butene, and in "acid leaching" to remove oxygenated impurities from stainless steel surfaces. Hydrofluoric acid is also used in the synthesis of many fluorinated organic compounds, such as Teflon (polytetrafluoroethylene) and Freon, a refrigerant. Click here and learn more.

Why choose us?

HRK Factory

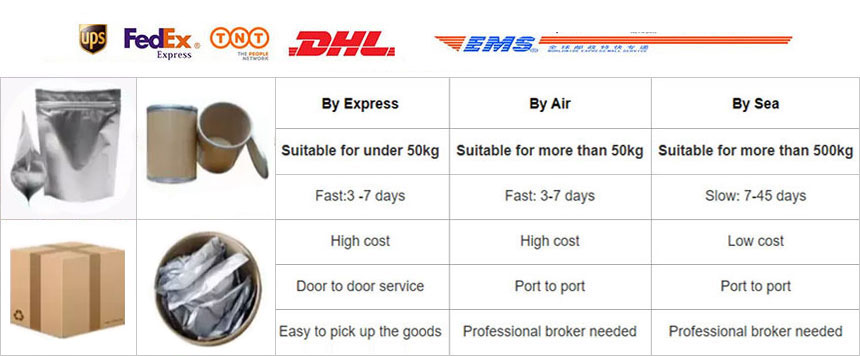
About Shipping
Pharmaceutical Intermediate manufacturers
©2023 Xi'an Henrikang Biotech Co., Ltd.,