





Related Attributes
Product details
Nickel sulfate CAS 7786-81-4 is available in anhydrous, hexahydrate and heptahydrate, and commercially available in hexahydrate, with α-type and β-type variants, the former with blue tetragonal crystals, the latter with green monoclinic crystals. According to the product classification, Nickel sulfate CAS 7786-81-4 can be divided into electroplating grade nickel sulfate and battery grade nickel sulfate, the former is used in electroplating industry and battery industry, is the main nickel salt of electroplating nickel and chemical nickel, and is the source of nickel metal ions, Nickel sulfate Powder can dissociate nickel ions and sulphate ions in the electroplating process; Battery grade nickel sulfate is one of the important raw materials for the production of ternary precursors, and different types of precursors have different nickel contents, which require different amounts of battery grade nickel sulfate.

Electroplating grade Nickel sulfate Powder and battery-grade nickel sulfate is the biggest difference between the cobalt content, battery-grade cobalt content requirements threshold is a little lower, the content requirements of only 0.4% (22.2% nickel, cobalt 0.4%), because nickel sulfate as a three-dimensional battery precursor raw materials manufacturing, also need to use cobalt sulfate and other cobalt-containing salts, and electroplating grade nickel sulfate application of the downstream surface treatment Industry, cobalt is as an impurity element, need to reduce its content, the cobalt content shall not exceed 0.05% (22.2% nickel, cobalt 0.05% Max), electroplating grade nickel sulfate is mainly used in the production of automobile wheels, appearance parts and other products.

Applications / Functions of Nickel sulfate Powder
Nickel sulfate Powder properties and stability
Nickel sulfate Raw Materials, also known as nickel alum, with the appearance of blue or green crystals, is an important nickel salt, soluble in ethanol and ammonia. In nature, there are three forms of nickel sulfate crystals: anhydrous, hexahydrate and heptahydrate, and the products sold in the market are mainly hexahydrate, including α and β two types of crystals, α-type is blue-green tetragonal crystals, β-type is green transparent crystals, slightly soluble in alcohol, easily soluble in methanol, α-type containing 6 molecules of water of crystallization is blue-green tetragonal crystals, and it transforms into β-type green transparent crystals in 53°C. Stable at 40°C, room temperature, it is stable at room temperature.

Stable at 40 ℃, room temperature becomes blue opaque crystals; containing 7 portions of water of crystallization for the emerald green transparent crystals, sweet and astringent taste, slightly weathered, about 100 ℃ when the loss of 5 molecules of water of crystallization to become monohydrate, at 280 ℃ into a yellowish-green anhydrous material. Easily soluble in concentrated ammonia (generated nickel-ammonia ions), but the solubility in organic solvents is very small. 280 ℃ to lose all the water of crystallization, the decomposition began at 840 ℃, releasing nickel-ammonia ions.
840 ℃ began to decompose, releasing sulfur trioxide, into nickel oxide. Below 31.5 ℃ crystallization of nickel sulfate heptahydrate, heptahydrate green transparent crystals, sweet and astringent taste, slightly easy to weather, relative density 1.948. melting point 98 ~ 100 ℃. 103 ℃ when the loss of 6 water of crystallization. Soluble in water and ethanol, very easy to deliquesce.
Containing 6 molecules of water of crystallization of α-type blue-green tetragonal crystals, at 53 ℃ transformed into β-type green transparent crystals. 40 ℃ stable, room temperature becomes blue opaque crystals. Containing 7 parts of water of crystallization is bright green transparent crystals. Sweet and astringent flavor. Slightly weathering. Lose 5 molecules of water of crystallization to become monohydrate at about 100℃, and become yellowish green anhydrous at 280℃. LD50 (rat, intraperitoneal) 500mg/kg, carcinogenic. Nickel sulfate has 3 kinds of anhydrous, hexahydrate and heptahydrate, with hexahydrate as the main material. Anhydrous material is yellow-green crystal, relative density 3.68, soluble in water, insoluble in ethanol, ether. 31.5 ~ 53.3 ℃ crystallization of nickel sulfate hexahydrate, hexahydrate is blue or emerald green fine-grained crystals, relative density 2.07.
Nickel sulfate Raw Materials is soluble in water and the aqueous solution is acidic. Easily soluble in concentrated ammonia (generating nickel ammonia ions), but in organic solvents solubility is very small (the common problem of sulfate, the lattice energy is too large under the circumstances). 280 ° C to lose all the water of crystallization, 840 ° C to start the decomposition, the release of sulfur trioxide, into nickel oxide. Below 31.5 ℃ crystallization of nickel sulfate heptahydrate, heptahydrate for green transparent crystals, sweet and astringent taste, slightly easy to weather, relative density 1.948. melting point 98 ~ 100 ℃. 103 ℃ when the loss of 6 water of crystallization.
Nickel sulfate Powder is soluble in water and ethanol, very easy to deliquesce. Nickel sulfate contact with dust and organic matter, sometimes can cause combustion or explosion. Toxic, maximum permissible concentration in air 0.5mg/m3.
Production method of Nickel sulfate Powder
The refining methods of Nickel sulfate Powder mainly include chemical precipitation method and extraction method. Chemical precipitation method, this kind of method through the crude nickel sulfate solution for sulfidation precipitation, oxidation precipitation, fluorine precipitation and then evaporation crystallization to get products.
The purpose of the present invention is to overcome the above shortcomings and provide a preparation method of refined nickel sulfate with low cost, clean production and good product quality.
The technical program of the present invention is:
First dissolve Nickel sulfate Powder with water to make crude nickel sulfate solution, add hydrogen peroxide, air, nitric acid or chlorate as oxidizing agent to the crude nickel sulfate solution, stir and heat, add alkali to adjust the pH value of the solution to 1.0-4.0, and react under the condition of 0°C-100°C for 0.5 After 0.5-8 hours, filter to get the iron and arsenic removal after liquid;
Then add hydrogen fluoride, ammonium fluoride or sodium fluoride as fluorinating agent in the after-liquid of removing iron and arsenic, stir and heat, add alkali to adjust the pH value of the solution to 2.0-6.0, and react for 0.5-8 hours under the condition of 0°C-100°C, and filter to get the after-liquid of removing calcium and magnesium;
In addition to calcium and magnesium in addition to saponification extractant, the use of alkali to adjust the solution pH 1.0-6.0, the organic phase and solution phase volume ratio (i.e., O / A) of 2:1-1:8 when the use of 1-4 extraction to remove copper and zinc and other impurities to get in addition to copper and zinc in addition to zinc after the liquid, the organic phase regeneration back to the production of saponification The organic phase is regenerated and returned to the preparation of saponification extractant, and the post-copper and zinc solution enters the subsequent process; the extractant used is bis (2-ethylhexyl) phosphonic acid (i.e., P204) or 2-ethylhexylphosphonic acid 2-ethylhexyl ester (i.e., P507); the said saponification extractant is the nickel salt of P204 or P507, and the method of saponification is to add nickel sulfate solution to nickel sulfate solution, and add nickel salts of P204 or P507 to nickel sulfate solution. Nickel sulfate solution, add extractant, add alkali to adjust the solution pH value of 5.0-8.0, extraction to get the saponification extractant; the use of saponification extractant extraction of copper and zinc extraction level 1-4; the use of pure nickel sulfate solution washing loaded with nickel organic phase, and the number of its washing level is 1-4 levels.
Said base is an alkali metal hydroxide, an alkali metal oxide or an aqueous solution of an alkali metal hydroxide, an aqueous solution of an alkali metal oxide.
After removing copper and zinc the liquid is added to a kerosene solution of the extractant, the pH of the solution is adjusted to 5.0-8.0 using alkali, and the nickel in the solution is extracted to obtain the loaded nickel organic phase. The loaded nickel organic phase is washed with pure nickel sulfate solution to remove the sodium, and the washed loaded nickel organic phase is back-extracted with 0.5 mol-L-1-6 mol-L-1 dilute sulfuric acid to obtain a purified nickel sulfate solution;
The purified nickel sulfate solution is heated and evaporated, cooled and crystallized, filtered to obtain emerald green crystals, and the crystals are dried to obtain a refined nickel sulfate product.
The advantages of the present invention are:
Why choose us?

HRK Factory

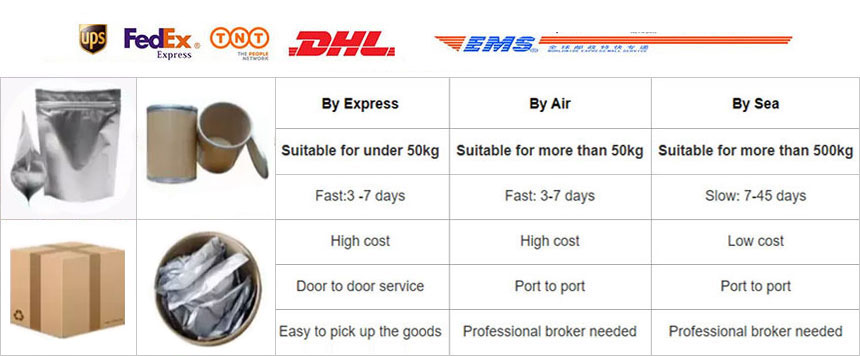
About Shipping
Pharmaceutical Intermediate manufacturers
©2023 Xi'an Henrikang Biotech Co., Ltd.,