





Related Attributes
Product details
Tetraethylammonium hydroxide liquid is a kind of organic quaternary ammonium base, the commodity mostly exists in the form of methanol and aqueous solution, colorless to light yellow liquid at room temperature, with strong alkali, can absorb carbon dioxide in the air, and decompose by heating.Tetraethylammonium hydroxide Raw Tetraethylammonium hydroxide CAS 77-98-5 is used as phase transfer catalyst, template agent for molecular sieve synthesis, cleaning agent, and decontamination agent in petroleum industry, etc. It can be used as phase transfer catalyst, template agent for molecular sieve synthesis, cleaning agent, and decontamination agent in petroleum industry. Tetraethylammonium hydroxide CAS 77-98-5 was used as a phase transfer catalyst to synthesize N,N-diethylaniline from aniline and ethyl bromide at atmospheric pressure.

Applications / Functions of Tetraethylammonium hydroxide liquid
Tetraethylammonium hydroxide Raw Materials are used as phase transfer catalysts, templates for molecular sieve synthesis and cleaning agents.
Phase transfer catalyst or electronics industry

Physicochemical Property of Tetraethylammonium hydroxide liquid
Density 1.041
Boiling point 110°C
Flash point 11°C
Water solubility SOLUBLE

Stable, but absorbs carbon dioxide from the air
Sensitivity Air Sensitive
production methodprocess of Tetraethylammonium hydroxide liquid
Tetraethylammonium hydroxide CAS 77-98-5 is prepared by electrolysis, the principle is that the aqueous solution of tetraethylammonium chloride in the anode chamber of electrolysis tank, under the action of electric field force, the chlorine ions in the solution migrate to the anode direction until they are discharged on the anode and precipitate chlorine gas. At the same time, due to the selective permeability of ionic membrane, chlorine ions can not diffuse through the ion exchange membrane, only tetraethylammonium ions can selectively permeate into the cathode chamber, and enriched in it. Water molecules in the cathode chamber of the electrolyzer are decomposed into hydrogen and hydroxide ions on the cathode. The latter happens to combine with the tetraethylammonium ion migrating from the anode chamber to form tetraethylammonium hydroxide. The concentration of tetraethylammonium hydroxide increases with the increase of energization, and finally reaches the expected crude concentration. The anode electrochemical reaction is: (C2H5)4NCl→(C2H5)4N++Clˉ2Clˉ-2e→Cl2↑ The cathode electrochemical reaction is: H2O→H++OHˉ(C2H5)4++OHˉ→(C2H5)4OH2H+2e→H2↑ Total reaction is: 2(C2H5)4NCl+2H2O→ 2(C2H5)4OH+H2↑+Cl2↑The hydrogen generated in the electrolysis method is vented, and the chlorine generated is absorbed with lye to generate sodium hypochlorite, which is the main raw material for bleaching powder. Therefore, this method to prepare Tetraethylammonium hydroxide liquid is simple, high purity and no environmental pollution.
Why choose us?

HRK Factory

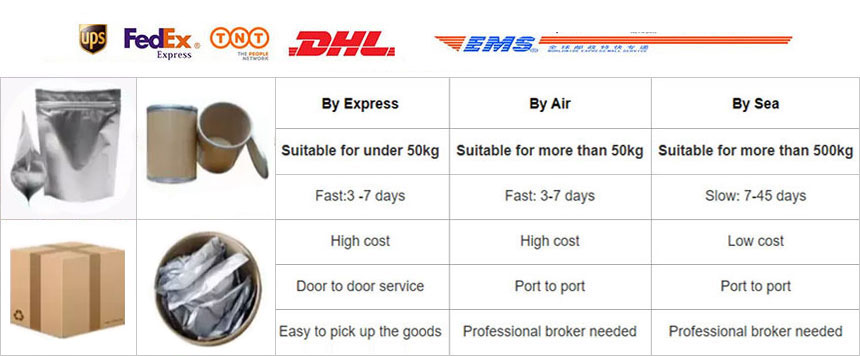
About Shipping
Pharmaceutical Intermediate manufacturers
©2023 Xi'an Henrikang Biotech Co., Ltd.,