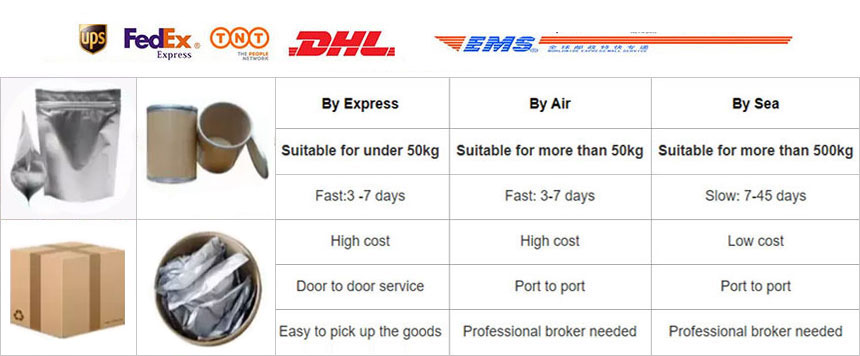
Phone: 86-29-89601602
Mail: sales27@interlgroup.com
Add: Room 305 , 3/F , Haipai Decoration Office Building , Yudu Avenue , Yuncheng , Shanxi
Posaconazole powder Raw Materials CAS 171228-49-2
Product Overview:
Posaconazole powder can effectively prevent invasive fungal infections. At present, although the injectable formulation of posaconazole has been approved by the FDA (approved in March 2014), its status as the main prophylactic drug has not changed. Posaconazole exerts its antifungal activity by inhibiting the biosynthesis of ergosterol. Ergosterol is an important component of fungal cell membrane to maintain the structural integrity and function of some membrane-associated proteins, and Posaconazole is also an essential trace substance in fungal cell cycle.
Posaconazole powder Raw Materials CAS 171228-49-2 Attributes
MF:C37H42F2N8O4
MW:700.78
EINECS:682-747-8
Specification:Posaconazole powder
Sample:Posaconazole powder
Brand:Posaconazole powder
Appearance: powder
Storage: Cool Dry Place
Brand: chemical Raw Materials
Shelf Life: 2 Years
Test Method: HPLC
Posaconazole powder Raw Materials CAS 171228-49-2 Details
Uses and synthesis of Posaconazole powder
Posaconazole powder can effectively prevent invasive fungal infections. At present, although the injectable formulation of posaconazole has been approved by the FDA (approved in March 2014), its status as the main prophylactic drug has not changed. Posaconazole exerts its antifungal activity by inhibiting the biosynthesis of ergosterol. Ergosterol is an important component of fungal cell membrane to maintain the structural integrity and function of some membrane-associated proteins, and Posaconazole is also an essential trace substance in fungal cell cycle.

Applications/Functions of Posaconazole powder
Posaconazole powder is used in patients with invasive aspergillosis refractory to amphotericin B or itraconazole or in patients who cannot tolerate treatment with these drugs.

Physicochemical Property of Posaconazole powder